
Soligenix, Inc. – A rare disease company with multiple near-term and potentially transformational catalysts
Drug development takes many years, but Soligenix has arrived in 2020 while remaining under the radar of many investors
Soligenix (Nasdaq: SNGX) is a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need. It has two unique areas of focus:
- a Specialized BioTherapeutics business segment, dedicated to the development of products for orphan diseases and areas of unmet medical need in oncology and inflammation; and
- a Public Health Solutions business segment, funded entirely by the US Government to the amount of approximately $70 million to date, developing vaccines and therapeutics for military and civilian applications in the areas of ricin exposure, emerging and antibiotic resistant infectious disease, and viral disease including Ebola, Marburg and COVID-19.
Rare Disease Pipeline
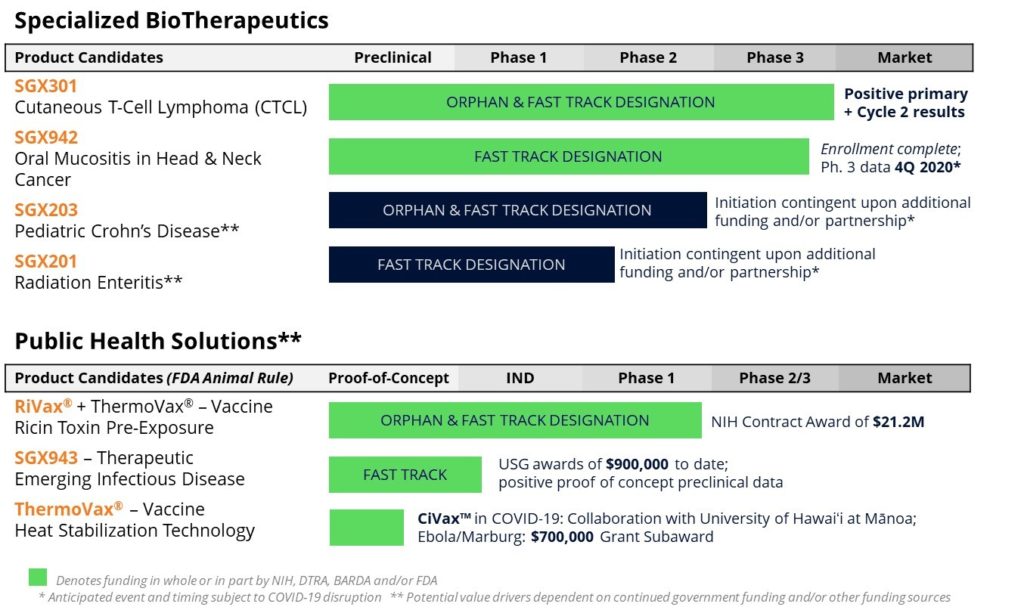
Under Specialized BioTherapeutics, the Company has two active Phase 3 clinical programs. The first is SGX301 (synthetic hypericin), a novel photodynamic therapy for the treatment of cutaneous T-cell lymphoma (CTCL), a rare class of non-Hodgkin’s lymphoma. In March of 2020, Soligenix achieved success when it announced positive and statistically significant results in the pivotal Phase 3 FLASH (“Fluorescent Light Activated Synthetic Hypericin”) study with SGX301. In the double-blind, placebo-controlled portion of the study (Cycle 1), a statistically significant treatment response (p=0.04) was achieved in the primary endpoint after 6 weeks of therapy (press release available here). This positive treatment response continued to dramatically improve with extended SGX301 treatment in the open-label treatment cycle, referred to as Cycle 2, with 12 weeks of therapy (p<0.0001 compared to placebo and p<0.0001 compared to 6-weeks treatment; press release available here). Next up will be the extended safety results from the optional, compassionate-use, 18 week treatment cycle (Cycle 3) expected in the fourth quarter of 2020. This clinical trial success was a tremendous accomplishment for the Company and demonstrates management’s expertise in executing rare disease studies. Soligenix will look to file for marketing authorization for SGX301 with the US Food and Drug Administration (FDA) where the global market potential for the product in CTCL is approximately $250 million.
The second Phase 3 program is SGX942 (dusquetide), a novel injectable drug that modulates the body’s own innate immune system to reduce inflammation, promote tissue healing, and clear infection. The pivotal Phase 3 clinical trial, referred to as the DOM-INNATE (“Dusquetide treatment in Oral Mucositis – by modulating INNATE Immunity”) study, treating oral mucositis in patients with head and neck cancer, completed patient enrollment in June (press release available here). The study successfully enrolled 268 subjects, following positive interim analysis, which included a prospectively defined, unblinded assessment of the study’s primary efficacy endpoint by an independent Data Monitoring Committee (DMC). With enrollment now completed, top-line results are expected in the fourth quarter of 2020. Assuming clinical trial success, Soligenix will begin preparing a marketing authorization for SGX942 where the global market potential for the product in oral mucositis could exceed $500 million.
Under its Public Health Solutions business segment, Soligenix continue to advance its work with the University of Hawaiʻi at Mānoa (UH Mānoa) and Hawaii Biotech Inc. (HBI) on heat stable filovirus vaccines (protecting against viruses such as Ebola and Marburg). They also extended this program with UH Mānoa to the development of vaccines to potentially combat coronaviruses, including SARS-CoV-2, the cause of COVID-19 (recent investor information session available here). The Company continues to support its FDA Fast Tracked (press release available here) heat stable ricin vaccine, RiVax®, with a National Institute of Allergy and Infectious Disease contract award of $21.2 million.
With over $11 million in cash as of the second quarter 10Q financial filing, not including its non-dilutive government funding, along with the at-the-market (ATM) sales issuance agreement with B. Riley FBR, Inc. to judiciously supplement its cash runway as needed, Soligenix anticipates having sufficient capital to achieve multiple inflection points across its rare disease pipeline, including top-line results in our SGX942 Phase 3 clinical trial in oral mucositis. The Company also continues to have ongoing confidential business development discussions, which may lead to more favorable capital inflows, including the potential to receive additional non-dilutive funding.
Of final note is Soligenix’s recent membership into the Russell Microcap® Index, which remains in place for one year and means automatic inclusion in the appropriate growth and value style indexes. FTSE Russell determines membership for its Russell indexes primarily by objective, market-capitalization rankings and style attributes. Russell indexes are widely used by investment managers and institutional investors for index funds and as benchmarks for active investment strategies. Approximately $9 trillion in assets are benchmarked against Russell’s US indexes. For more information on the Russell Microcap® Index, go to the “Russell Reconstitution” section on the FTSE Russell website here.
For further information regarding Soligenix, Inc., please visit the Company’s website at www.soligenix.com.















